
Electron wave tomography:
One-electron atom
General Chemistry
http://quantum.bu.edu/notes/GeneralChemistry/OneElectronOrbitalTomography.html
Updated
Wednesday, November 5, 2008 10:31 AM
Copyright © 2006 Dan Dill (dan@bu.edu)
Department of Chemistry, Boston University, Boston MA 02215


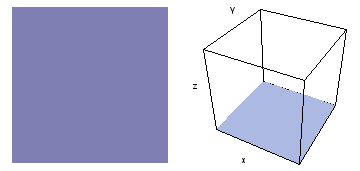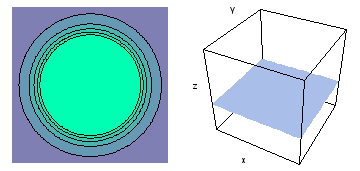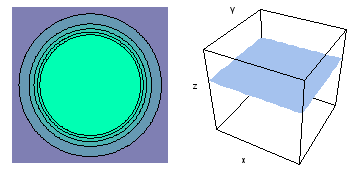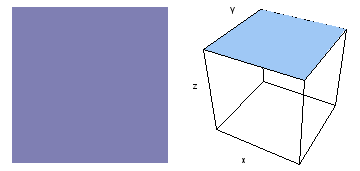
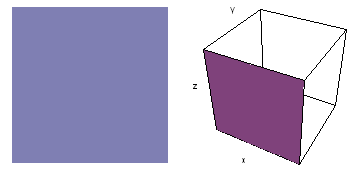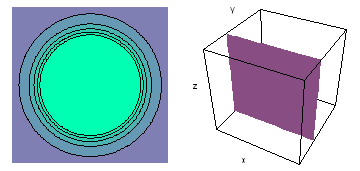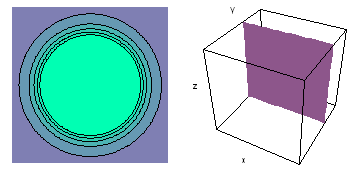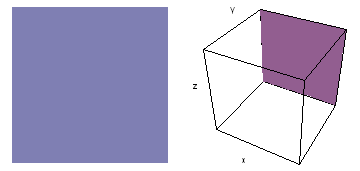
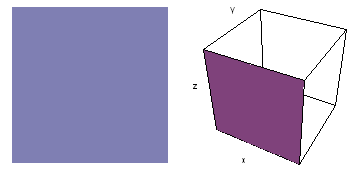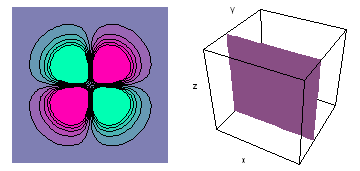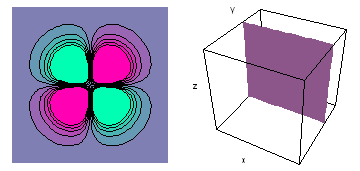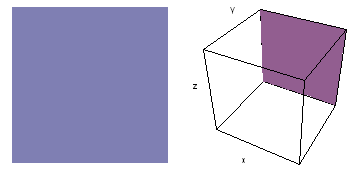
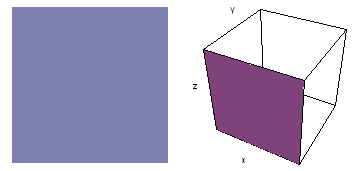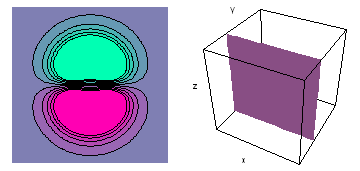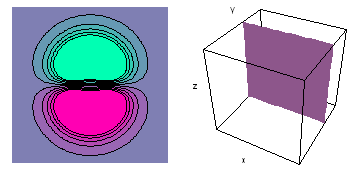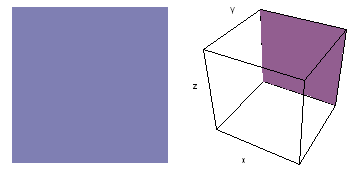
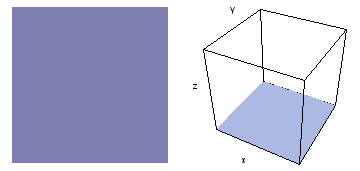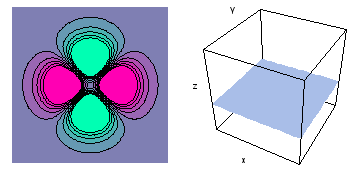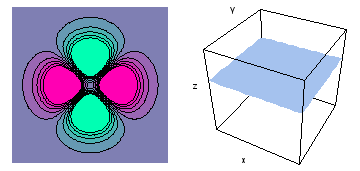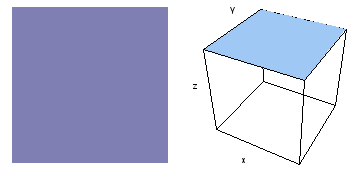
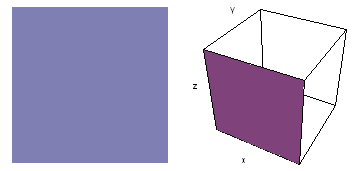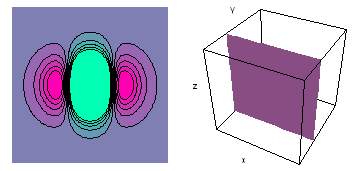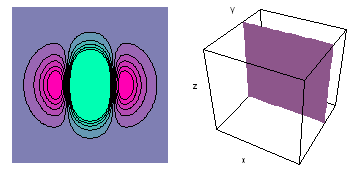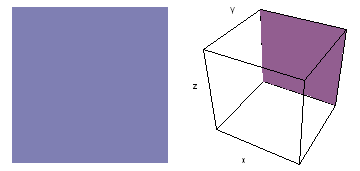
It is a challenge to visualize three dimensional electron waves (wavefunctions). One way to do it is to look at the electron wave values on a plane. The sets of two pairs of animated figures below do this on the xy (first pair) and xz (second pair) planes for a particular s, p or d electron wave of an electron in a hydrogen atom.
The left-hand animation of each pair is a contour plot of the electron wave values (green denotes positive phase, red denotes negative phase) along the plane positioned as shown in the right-hand figure. The nucleus is at the center of the cube.
For each electron wave, see if you can determine the values of the quantum number n whether the angular function is s, px, py, pz, dx2y2, dxy, dyz, dzx, or dz2.
Electron wave 1
Electron wave 2
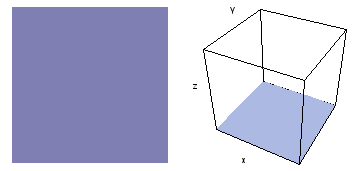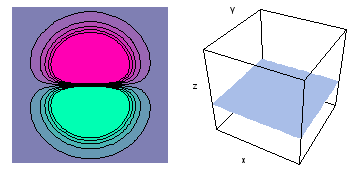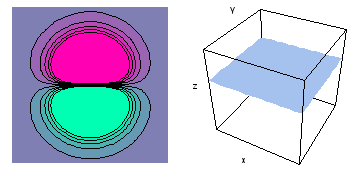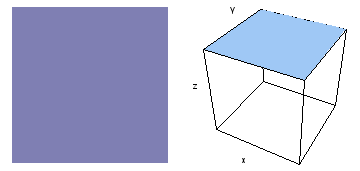
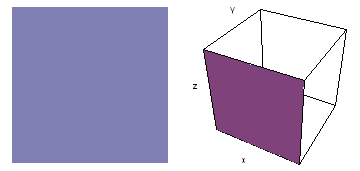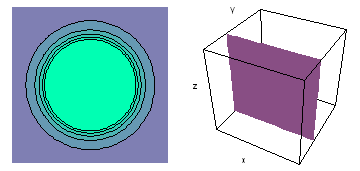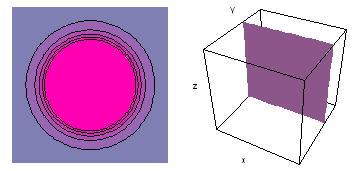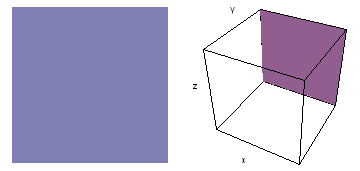
Electron wave 3
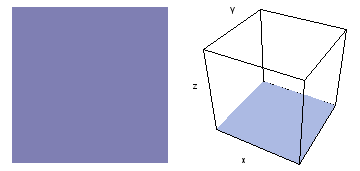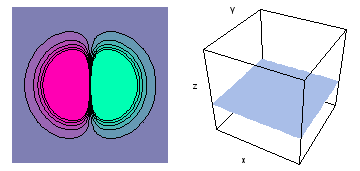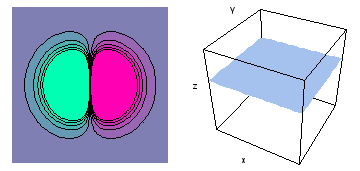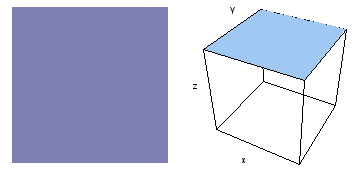
Electron wave 4
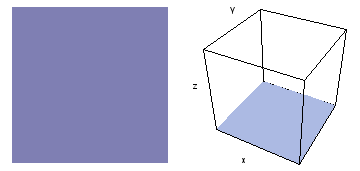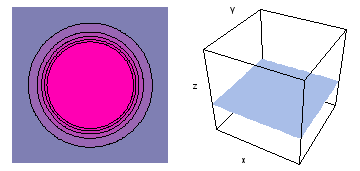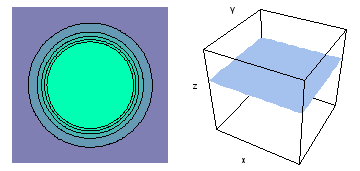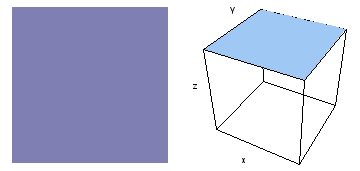
Electron wave 5
[ Top ]
http://quantum.bu.edu/notes/GeneralChemistry/OneElectronAtom.html
Updated
Wednesday, November 5, 2008 10:31 AM
Dan Dill (dan@bu.edu)