
Fermi holes and Fermi heaps
Notes on Quantum Mechanics
http://quantum.bu.edu/notes/QuantumMechanics/FermiHolesAndHeaps.html
Updated
Thursday, November 13, 2003 1:14 PM
Copyright © 2003 Dan
Dill (dan@bu.edu)
Department of
Chemistry, Boston University, Boston
MA 02215

You may have learned the "rule" that no more than two electrons can be in the same orbital. If you have, you may also have puzzled about why such a rule is so. If you have decided, like many people who have been presented with just the rule without any explanation, that it has to do with electrical repulsion—that it reflects the electrons repelling one another due to their electrical charge—then you are in for a neat surprise. The "rule" instead traces to a deep algebraic property of nature that has nothing whatsoever to do with the charge on electrons! Perhaps you, like me, will find it fascinating that such a crucial aspect of the world has such a subtle origin.
The essence is that many-electron wavefunctions must change sign when the labels on any two electrons are interchanged. This property is called antisymmetry, and its essential consequence is that electrons either stay out of one another's way, forming what is called a Fermi hole, or clump together, forming what is called a Fermi heap. Since electrons repel one another electrically, Fermi holes and Fermi heaps has drastic effects on the energy of many-electron atoms. The most profound result is the periodic properties of the elements.
Details of the origin and significance of Fermi holes and Fermi heaps are discussed elsewhere, but here are illustrations of Fermi hole and Fermi heaps in the carbon atom.
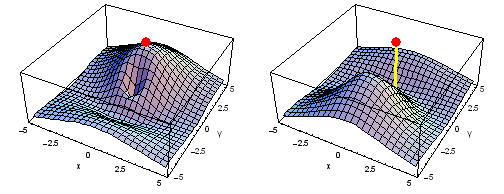
Carbon 1s2py Fermi heap (left) and Fermi hole (right). The post marks the position of one electron, at y = -2 a0 in the xy plane. The height of the surface is the probability density of the other electron. The Fermi heap shows the clumping of the two electrons, while the Fermi hole shows that the two electrons are never found in the same place.
Animation of the carbon 1s2py Fermi heap (left) and Fermi hole (right). The post marks the position of one electron. The animation shows the variation of the probability density for locations of one electron on a ring of radius 2 a0 in the xy plane.
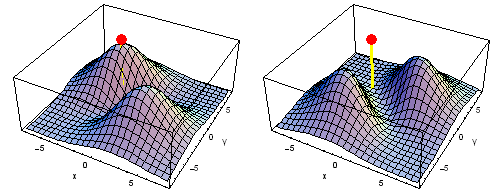
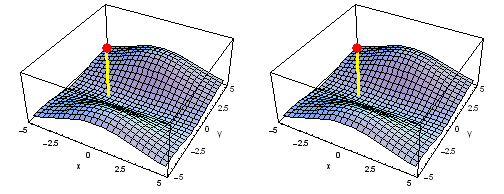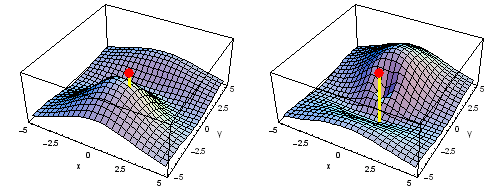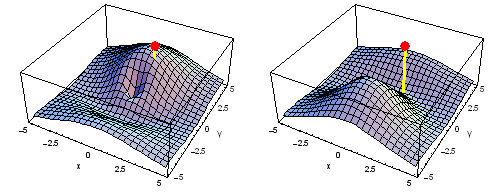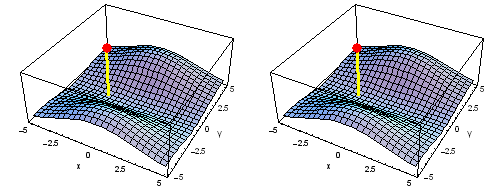
Carbon 2px2py Fermi heap (left) and Fermi hole (right). The post marks the position of one electron, at {x, y} = {2 sqrt(2), 2 sqrt(2)} a0 in the xy plane. The height of the surface is the probability density of the other electron. The Fermi heap shows the clumping of the two electrons, while the Fermi hole shows that the two electrons are never found in the same place.
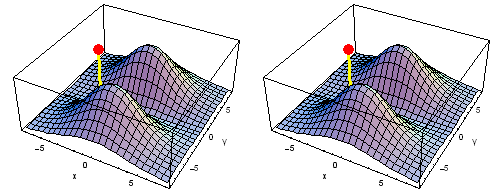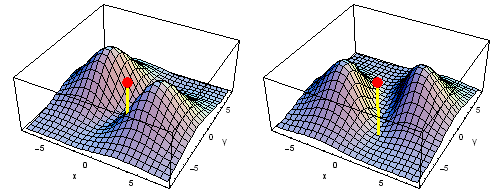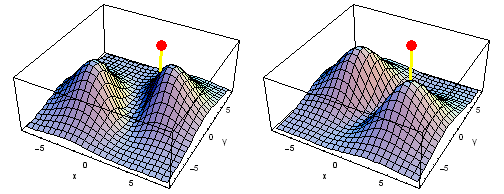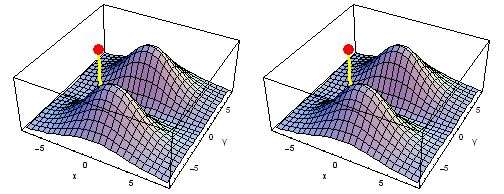
Animation of the carbon 2px2py Fermi heap (left) and Fermi hole (right). The post marks the position of one electron. The animation shows the variation of the probability density for locations of one electron on a ring of radius 4 a0 in the xy plane.
[ Top ]
http://quantum.bu.edu/notes/QuantumMechanics/FermiHolesAndHeaps.html
Updated
Thursday, November 13, 2003 1:14 PM
Dan
Dill (dan@bu.edu)